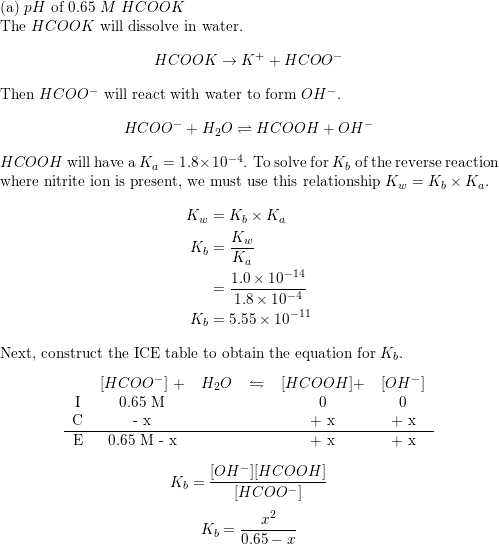Interconversion between CO2 and HCOOH under Basic Conditions Catalyzed by PdAu Nanoparticles Supported by Amine-Functionalized Reduced Graphene Oxide as a Dual Catalyst | ACS Catalysis

Effect of HCOOK/Ethanol on Fe/HUSY, Ni/HUSY, and Ni–Fe/HUSY Catalysts on Lignin Depolymerization to Benzyl Alcohols and Bioaromatics | ACS Omega
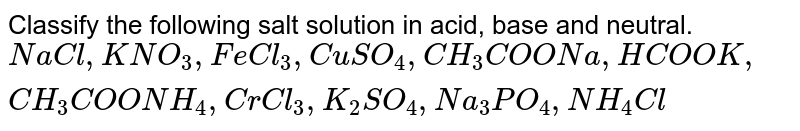
Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl
Steady continuous dosage of FA. Reaction conditions: HCOOH (5 mmol),... | Download Scientific Diagram

SOLVED: State whether aqueous solutions of the following salts produce acidic, basic or neutral solutions Justify your choice by referring to the specific properties of BOTH the cation and anion. You must

EHSQ (Environment,Health,Safety and Quality) : Question : Is HCOOK an acid or base or neutral ? Answer : HCOOK ( Potassium formate ) is base
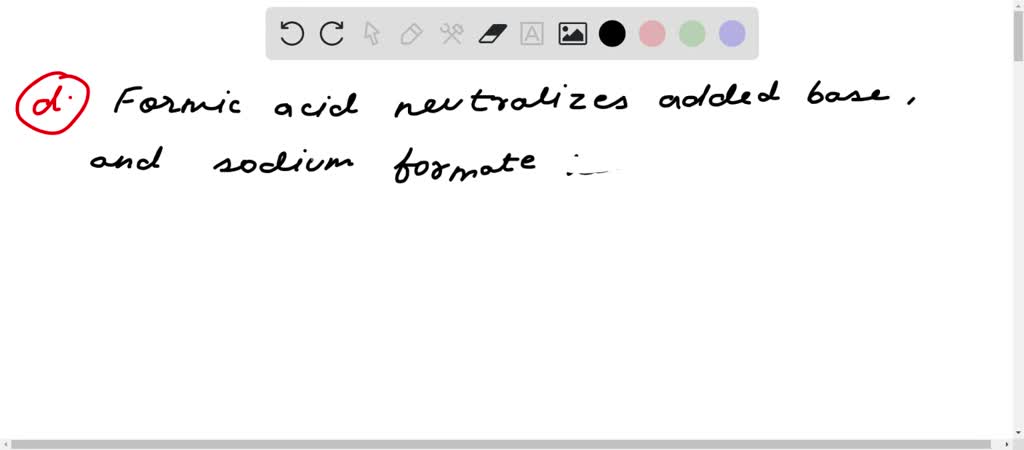
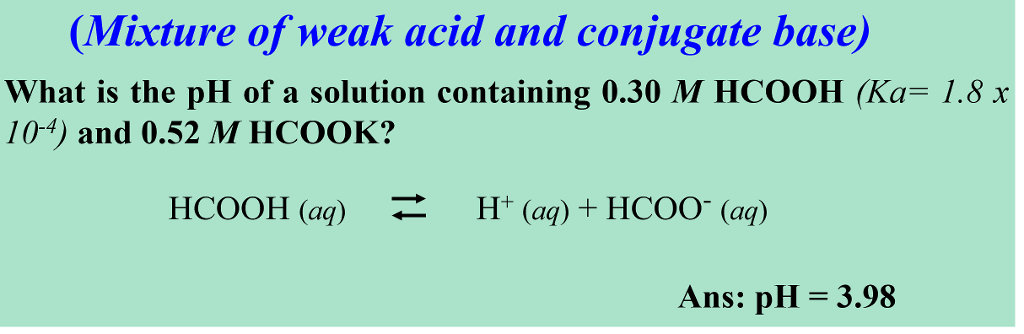
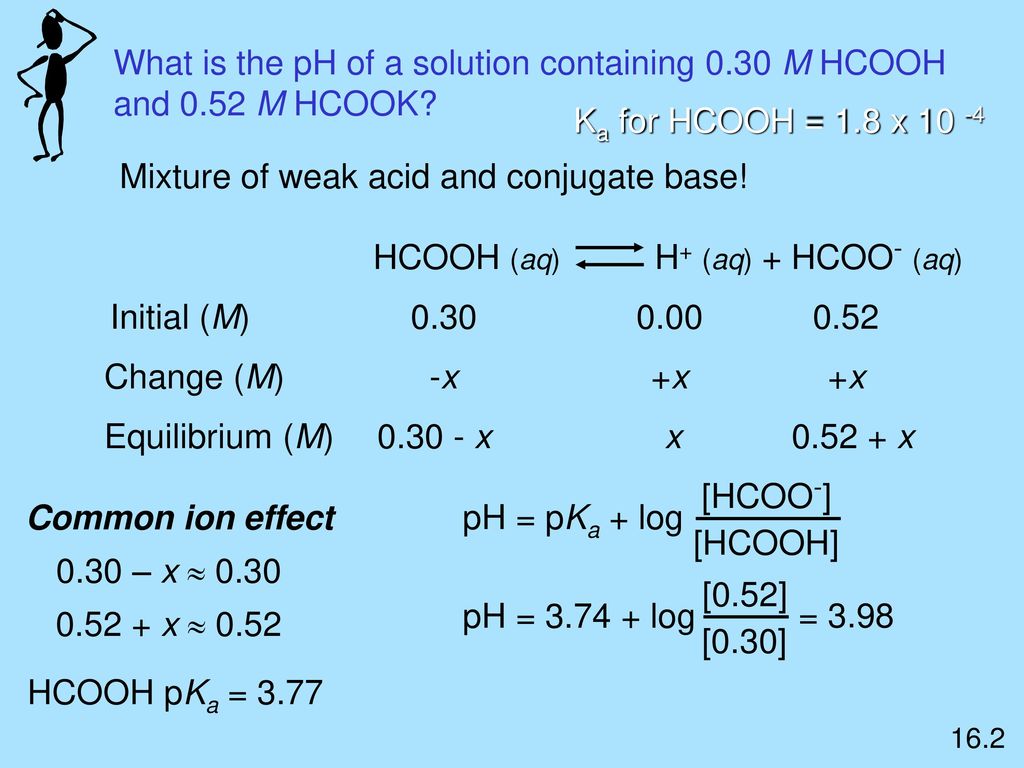
SOLVED: A buffer contains HCOOH (aq) and HCOOK (aq). Which statement correctly summarizes the action of this buffer? Both HCOOH (aq) and HCOOK (aq) neutralize added acid. Both HCOOH (aq) and HCOOK (

EHSQ (Environment,Health,Safety and Quality) : Question : Is HCOOK an acid or base or neutral ? Answer : HCOOK ( Potassium formate ) is base

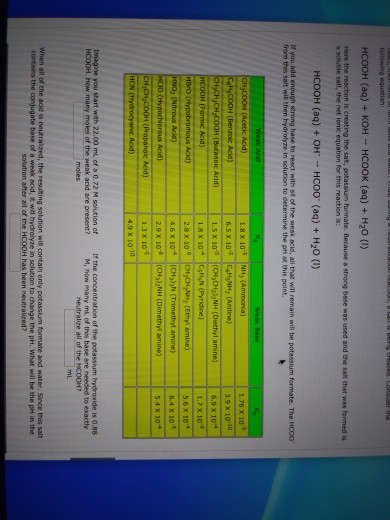
Why an aqueous solution of NH4Cl is acidic while that of HCOOK is basic - Chemistry - Ionic Equilibria - 16488273 | Meritnation.com

Acid-Base Equilibria Chapter HF( aq ) + H 2 O( l ) H 3 O + ( aq ) + F - ( aq ) Addition of NaF will shift the equilibrium to the _______because. - ppt download