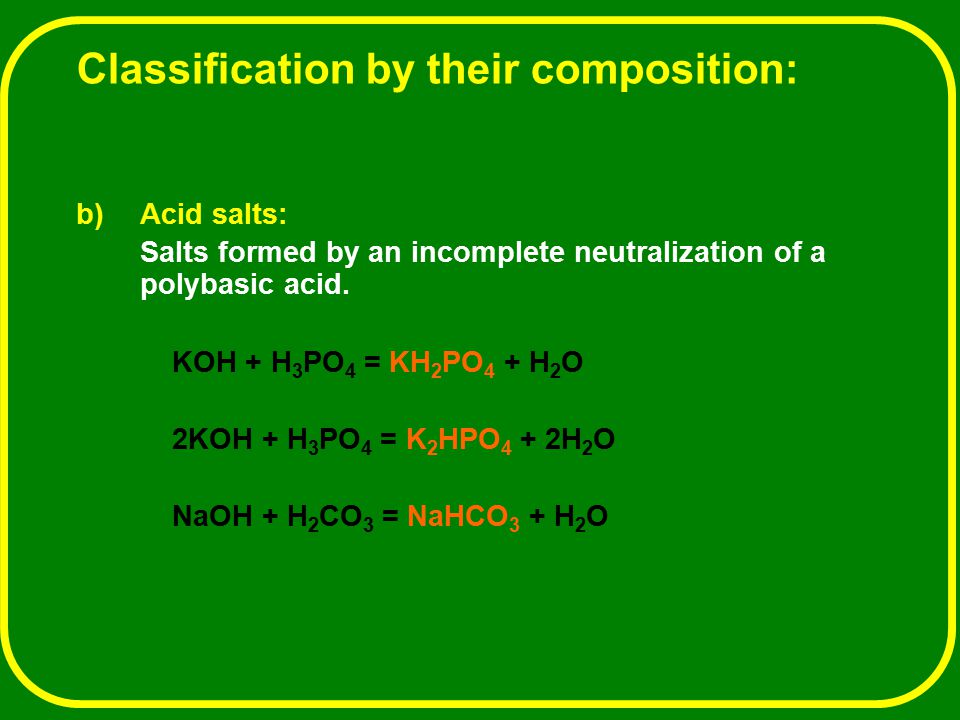
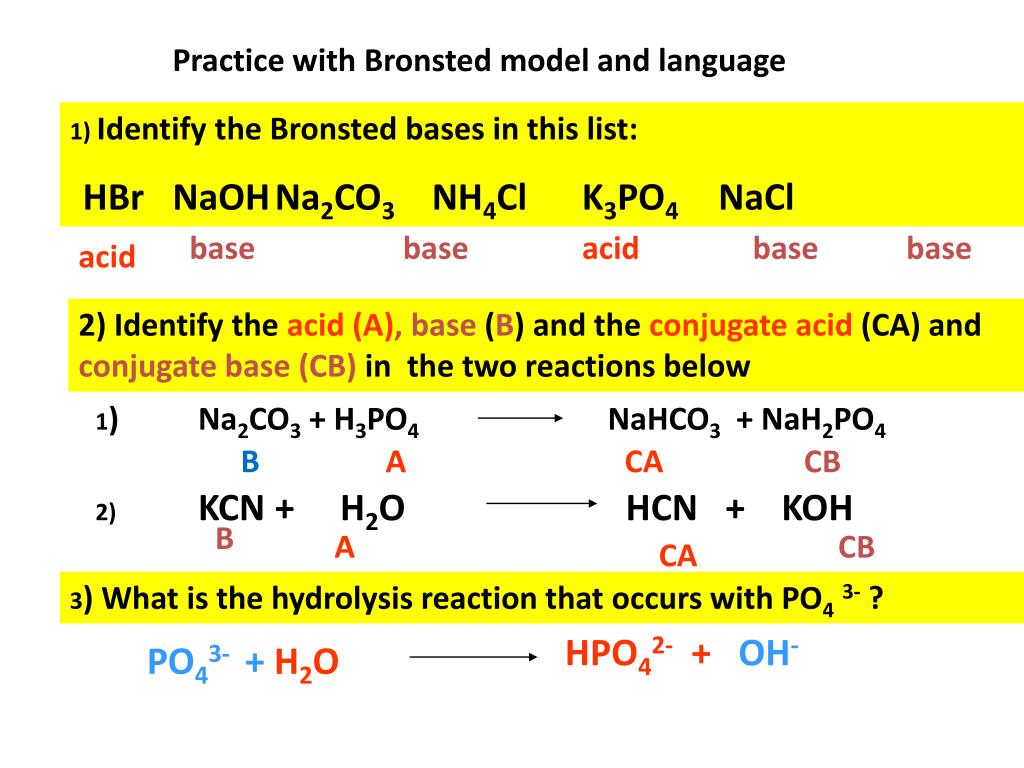
Potassium Phosphate as a High-Performance Solid Base in Phase-Transfer-Catalyzed Alkylation Reactions | Industrial & Engineering Chemistry Research

Air-Tolerant Direct Thiol Esterification with Carboxylic Acids Using Hydrosilane via Simple Inorganic Base Catalysis | The Journal of Organic Chemistry
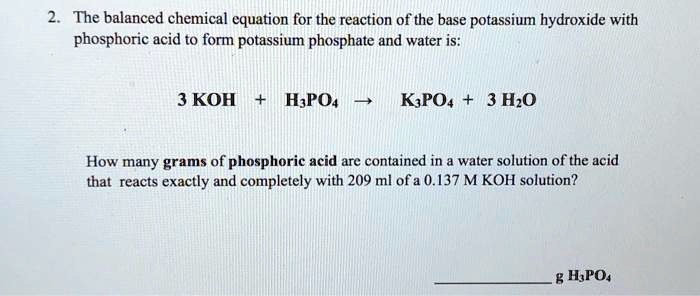
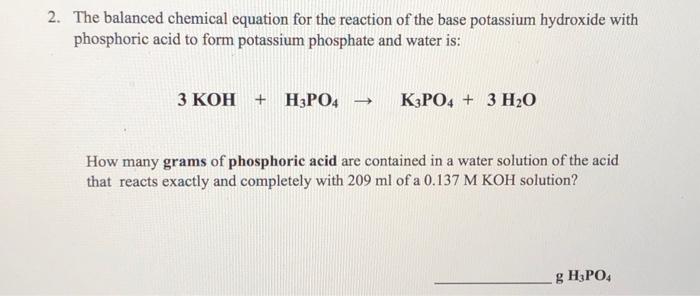
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH HPO4 KaPOs 3 HzO How many grams