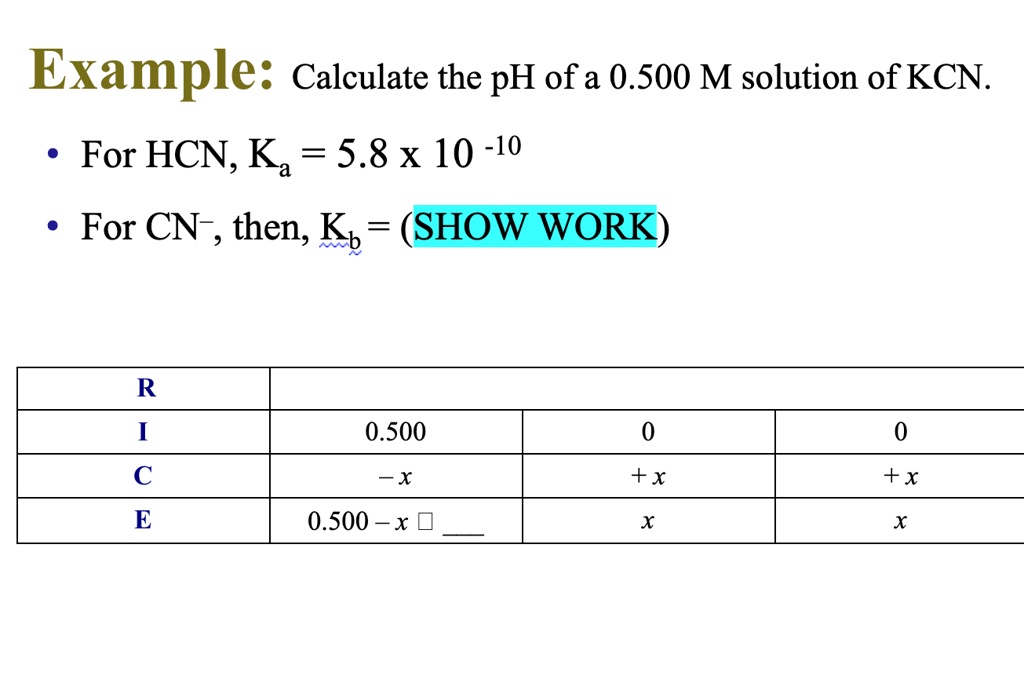
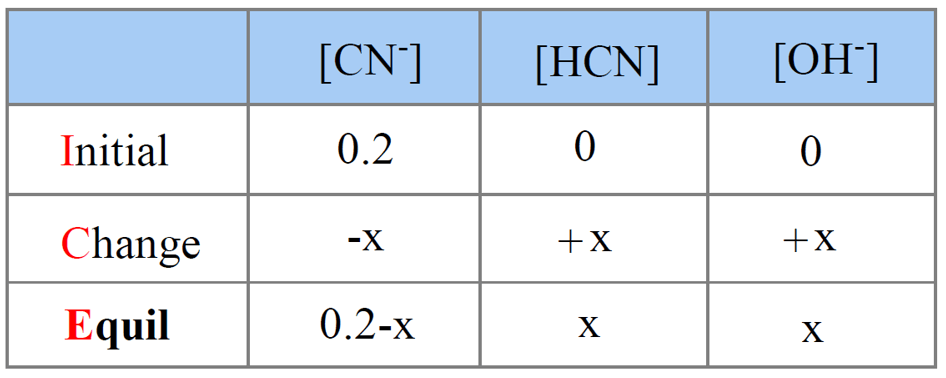
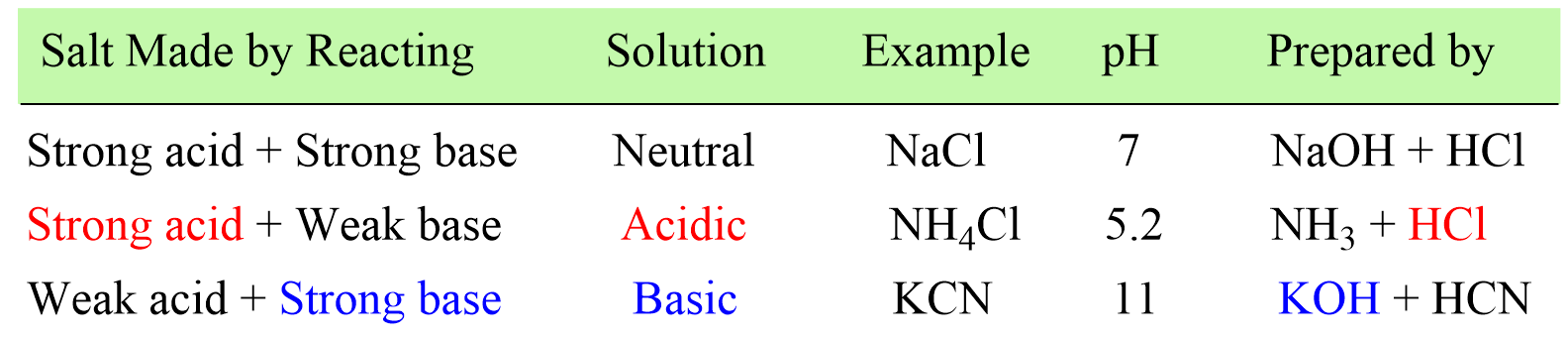
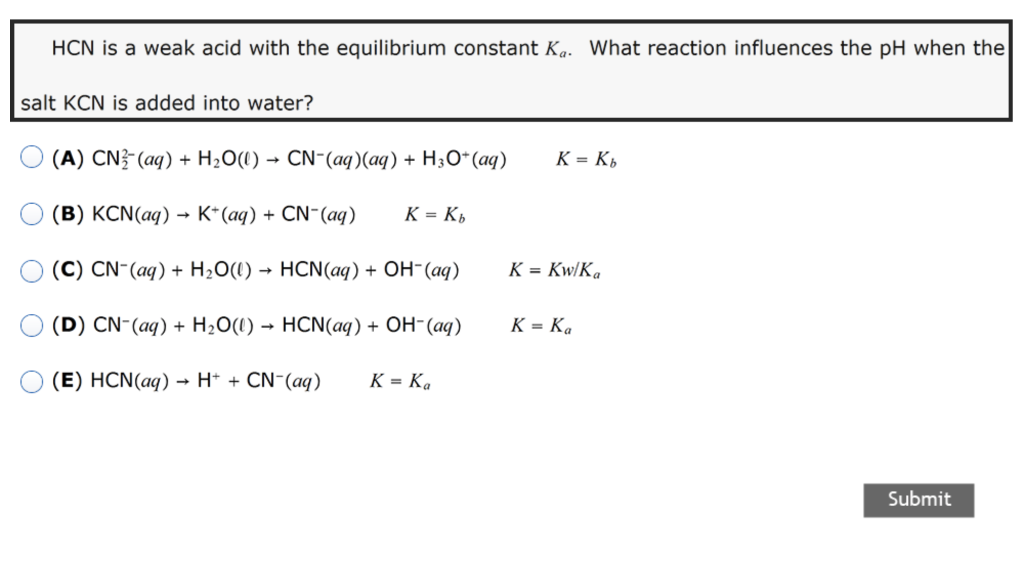
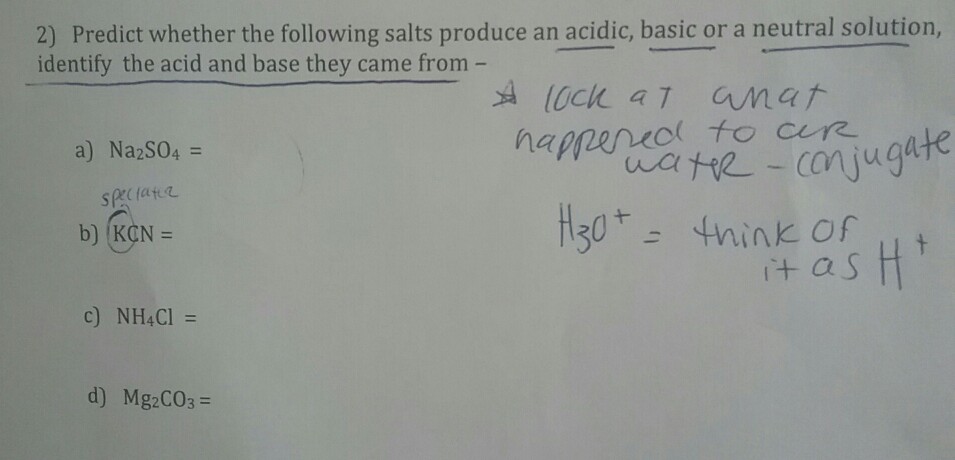pH of salt solutions 1.Salts derived from strong acids and strong bases These consist of cations from strong bases and the anions from. - ppt download

How to Determine if Salt is Acidic, Basic, or Neutral Example, Problem, Shortcut, Explained Question - YouTube

12) KCN (Potassium Cyanide) 1800's | Organic chemistry, Organic chemistry books, Organic chemistry reactions

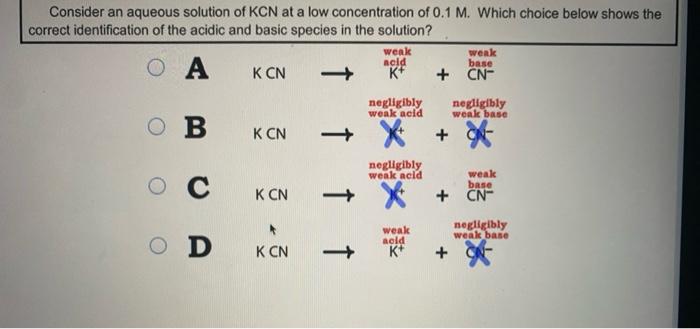
OneClass: Consider the following data on some weak acids and weak bases: acid base C0 name formula na...
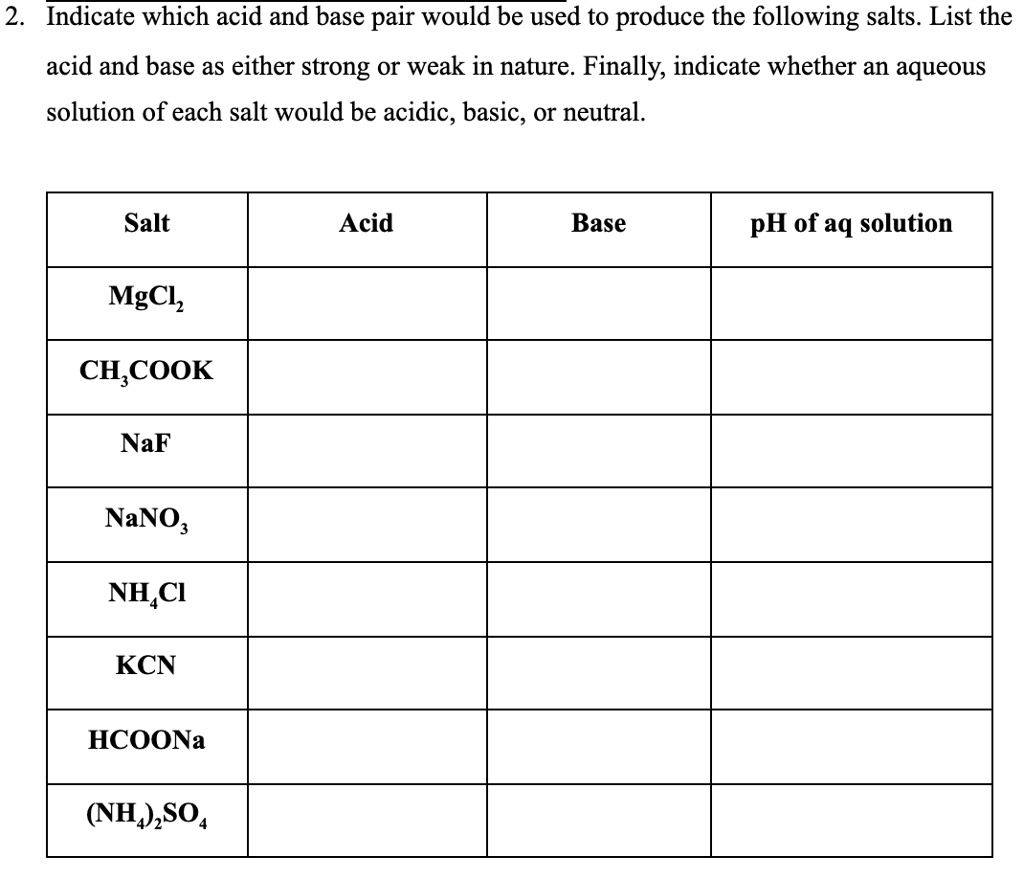
SOLVED: 2 Indicate which acid and base pair would be used to produce the following salts List the acid and base as either strong or weak in nature. Finally, indicate whether an

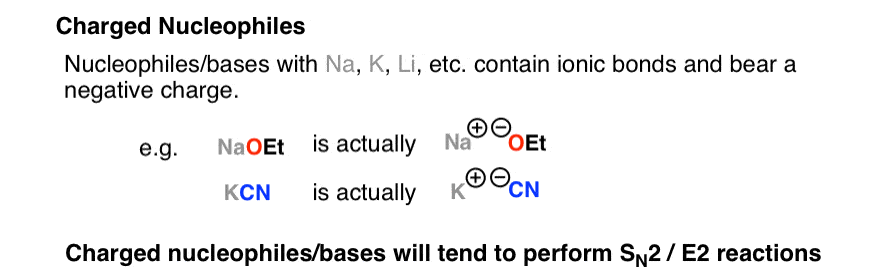
Acid-Base Properties of Salts. These salts simply dissociate in water: KCl(s) K + (aq) + Cl - (aq) - ppt download