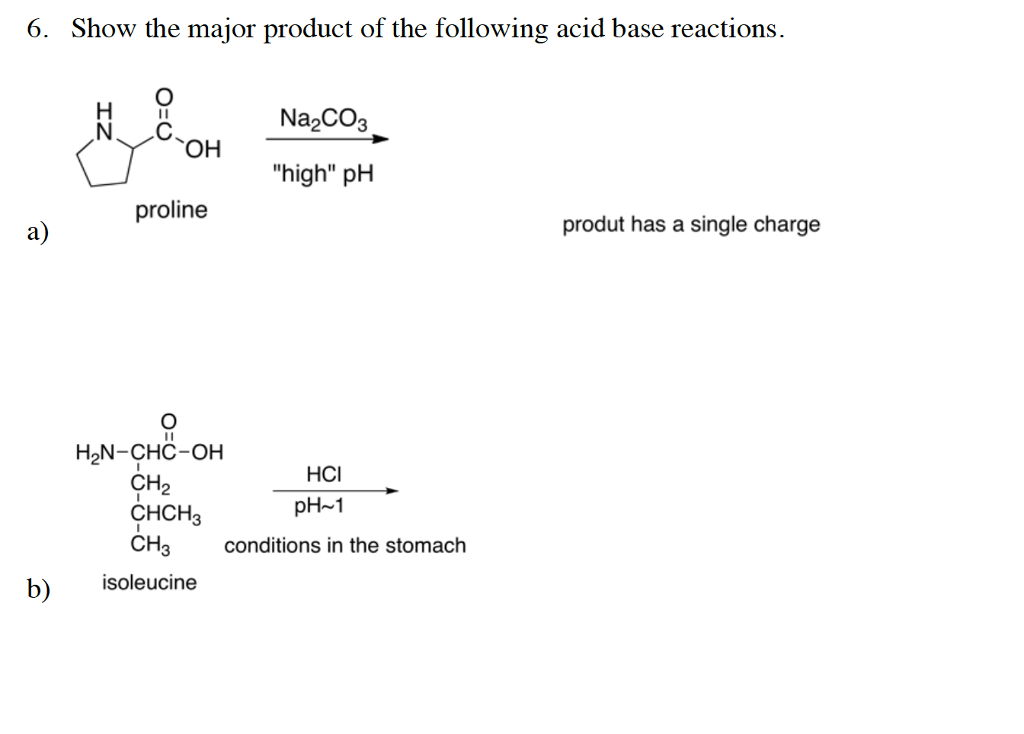
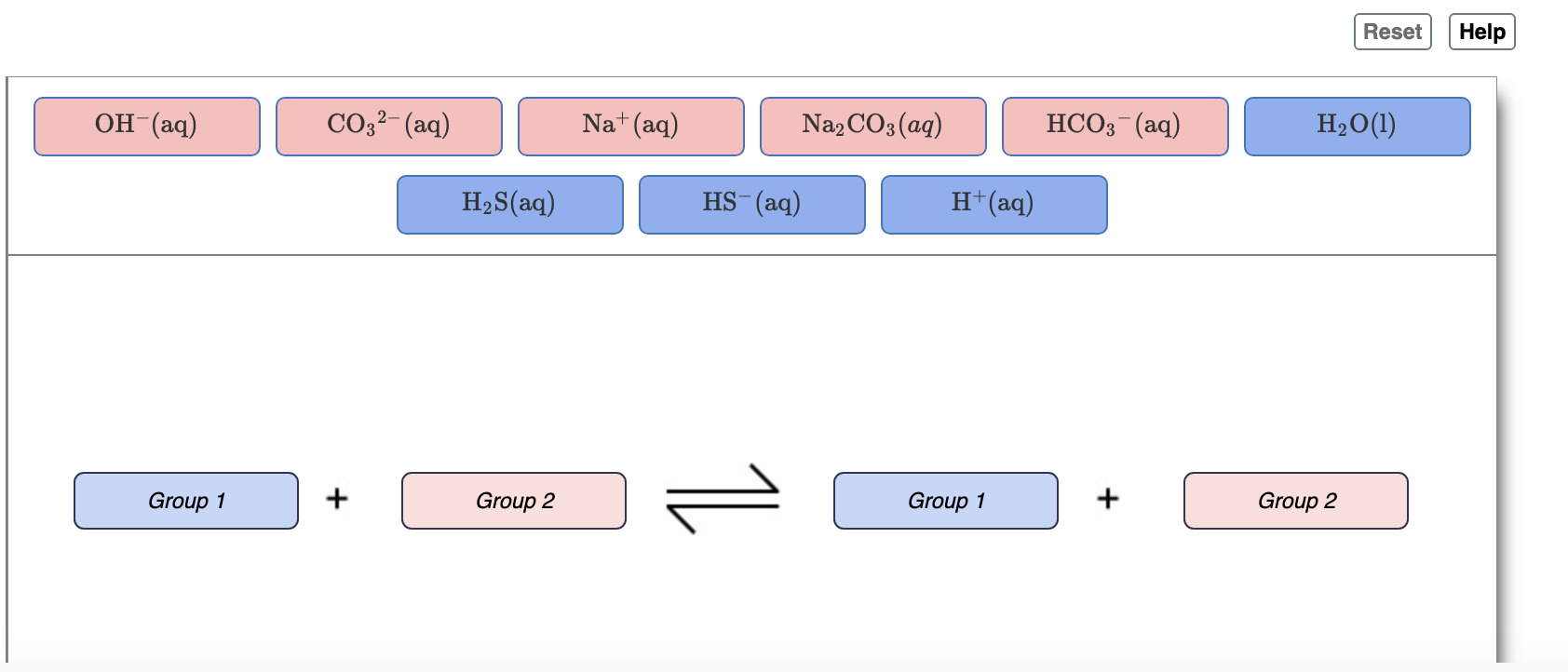
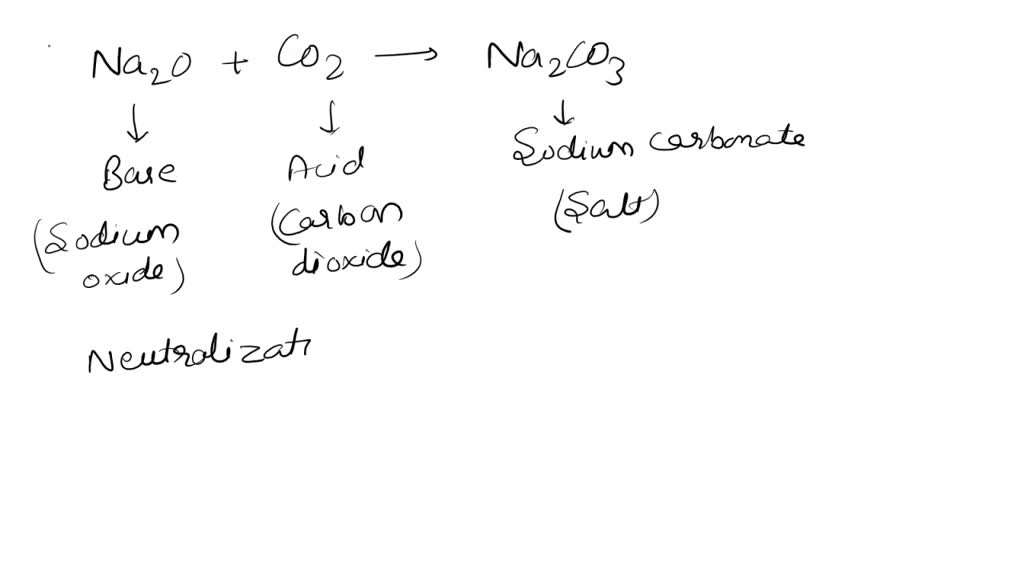
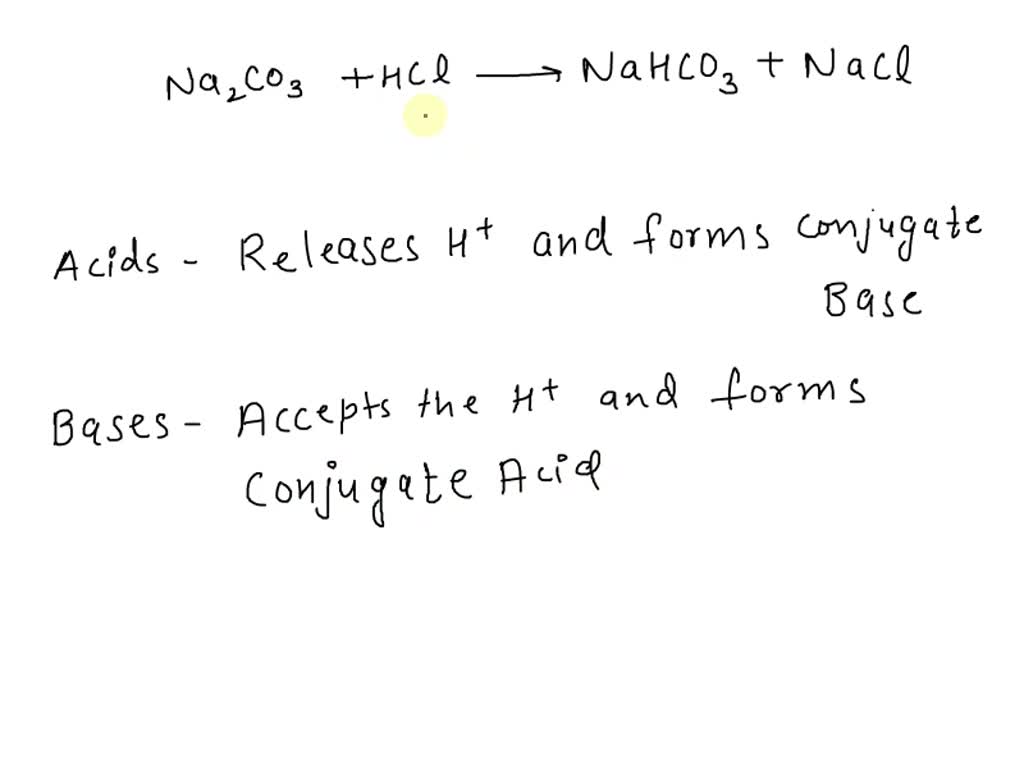
DOC) Standardization of a strong acid (HCl) with a weak base (Na2CO3).docx | Meharaj Ul Mahmmud - Academia.edu

SOLVED: Q1) A solution of Na2CO3 has a pH 12.50. Write the net ionic equation(hydrolysis) for the reaction which makes a solution of Na2CO3 basic. Make sure to not include spectator ions

Write a mechanism (using curved-arrow notation) for the deprotonation of tannins in base. Use Ar-OH as a generic form of a tannin and use sodium carbonate (Na2CO3) as the base. Balance the

Acid–base titration curves of the biomass pretreated with NaHCO3 sat.... | Download Scientific Diagram