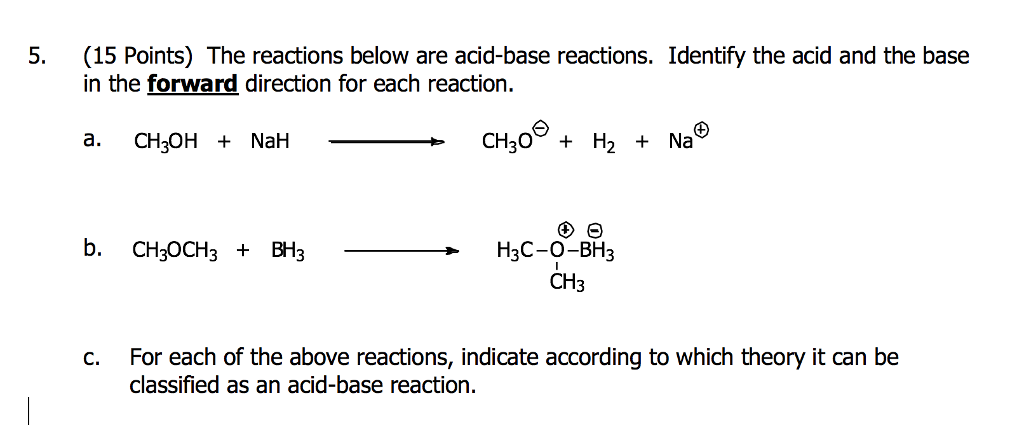
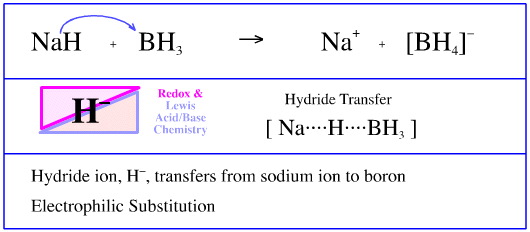
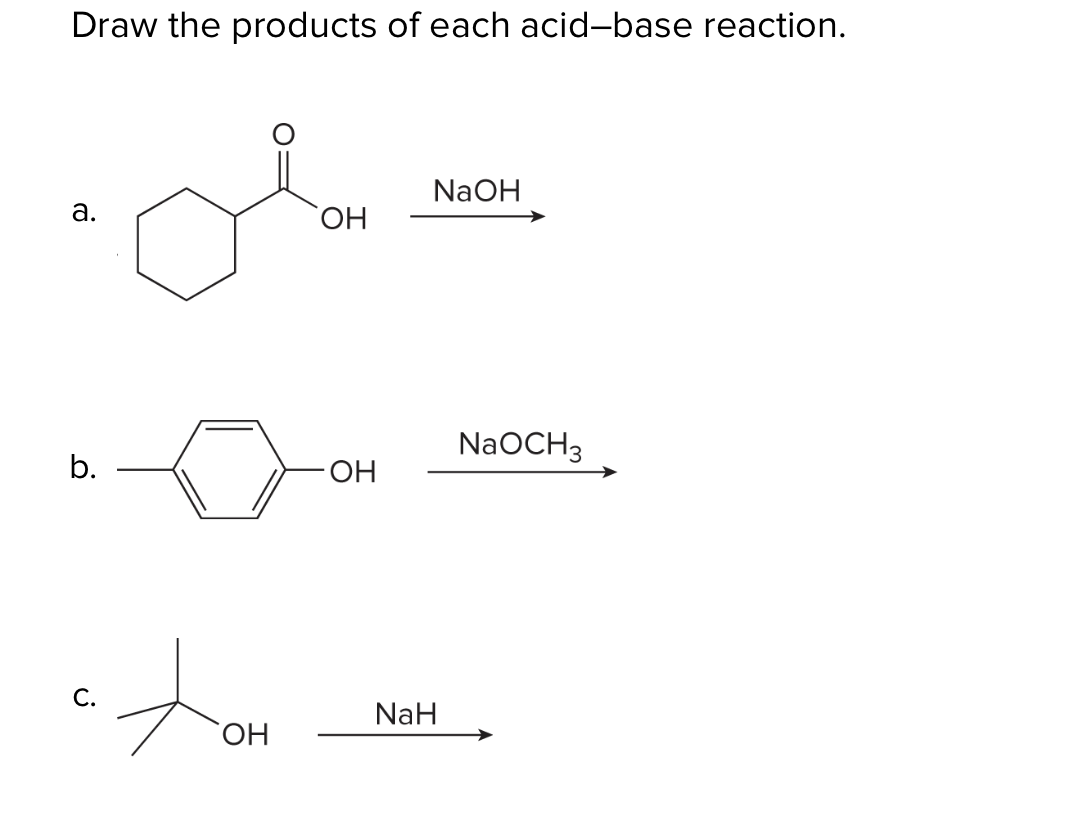
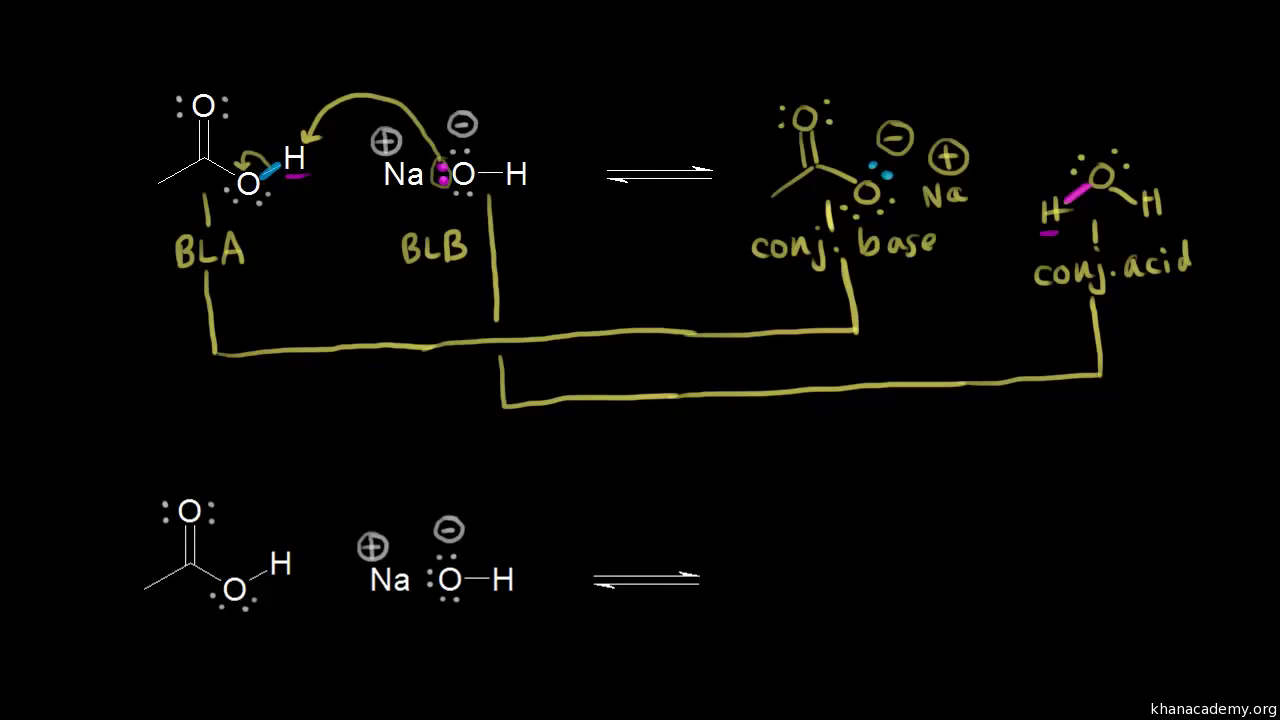
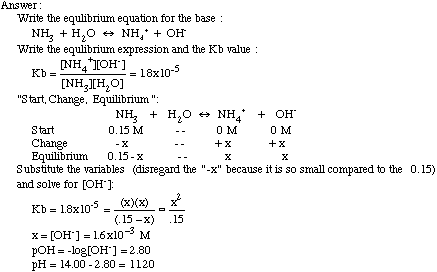SOLVED: Sodium hydride (NaH) is convenient source Of the hydride anion (H ) the conjugate base of Hz: In contrast t0 other hydride reagents that we will learn about next semester; NaH
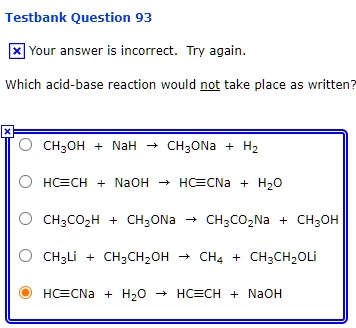
SOLVED: Testbank Question 93 Your answer incorrect Try again Which acid base reaction would not take place as written CHzOH Nah CHzONa HCCH NaOH HCCNa Hzo CH;COzH CHzONa CH;COzNa CHzOH CHzLi CHzCHzOH

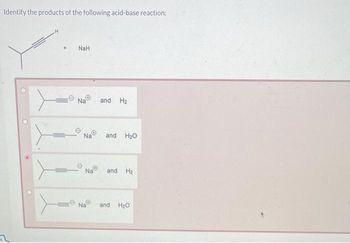
What product is formed when the following compound is treated with NaH? The following acid-base reactions were a step in a synthesis of a commercially available drug. | Homework.Study.com

organic chemistry - Can NaH open the epoxide ring to form alcohol? If so, how? - Chemistry Stack Exchange

What product is formed when the given compound is treated with NaH? The given acid-base reactions were a step in a synthesis of a commercially available drug. | Homework.Study.com

The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?
Welcome to Chem Zipper.com......: A 0.0258 M solution of the sodium salt, NaH of the weak monoprotic acid, HA has a pH of 9.65. Calculate Ka of the acid AH.

OneClass: Predict the structures of BOTH bronsted acid base reaction of NaH with typical thiol. What ...