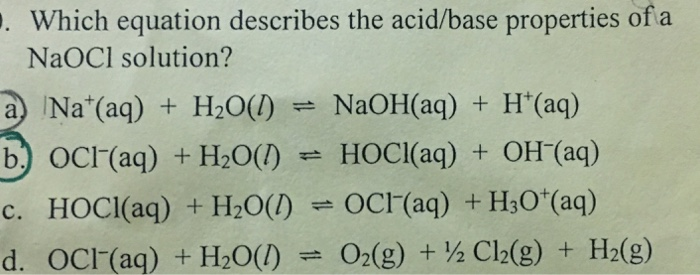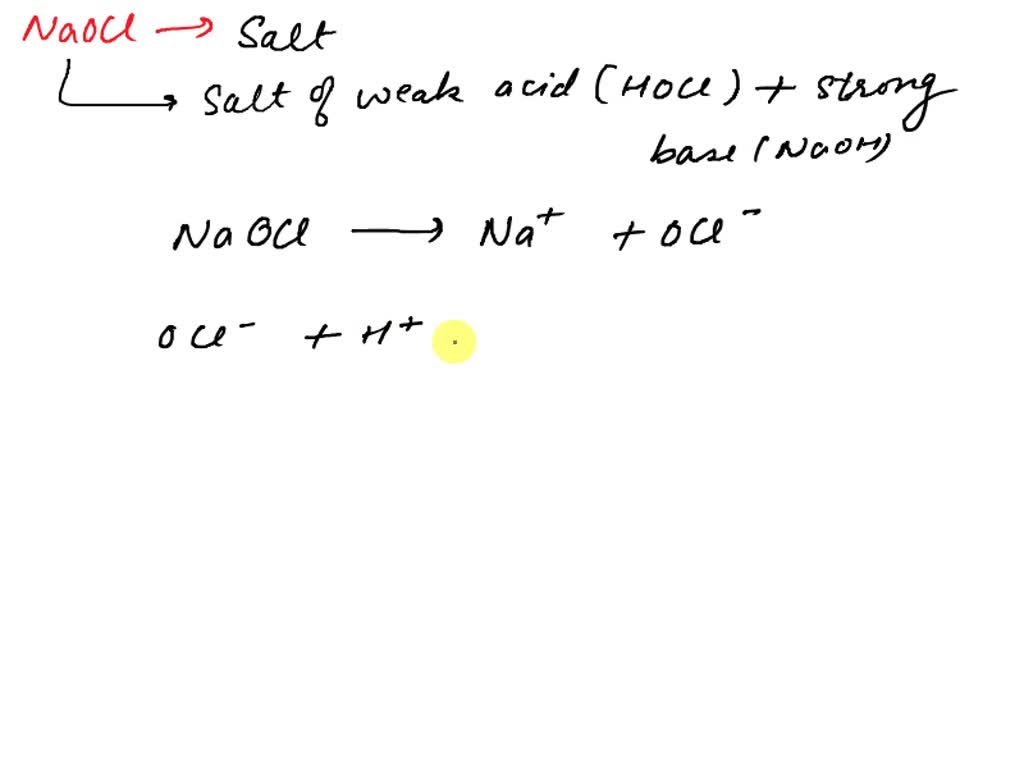
SOLVED: 14. Hypochlorous acid, HOCl(aq), is a weak acid with Ka = 2.9 x 10–8 at 25 oC. A chemist prepares a 0.020 M solution of sodium hypochlorite, NaOCl(aq) at 25 oC.

Why can water act as a base under acidic conditions in organic chemistry mechanisms? - Chemistry Stack Exchange

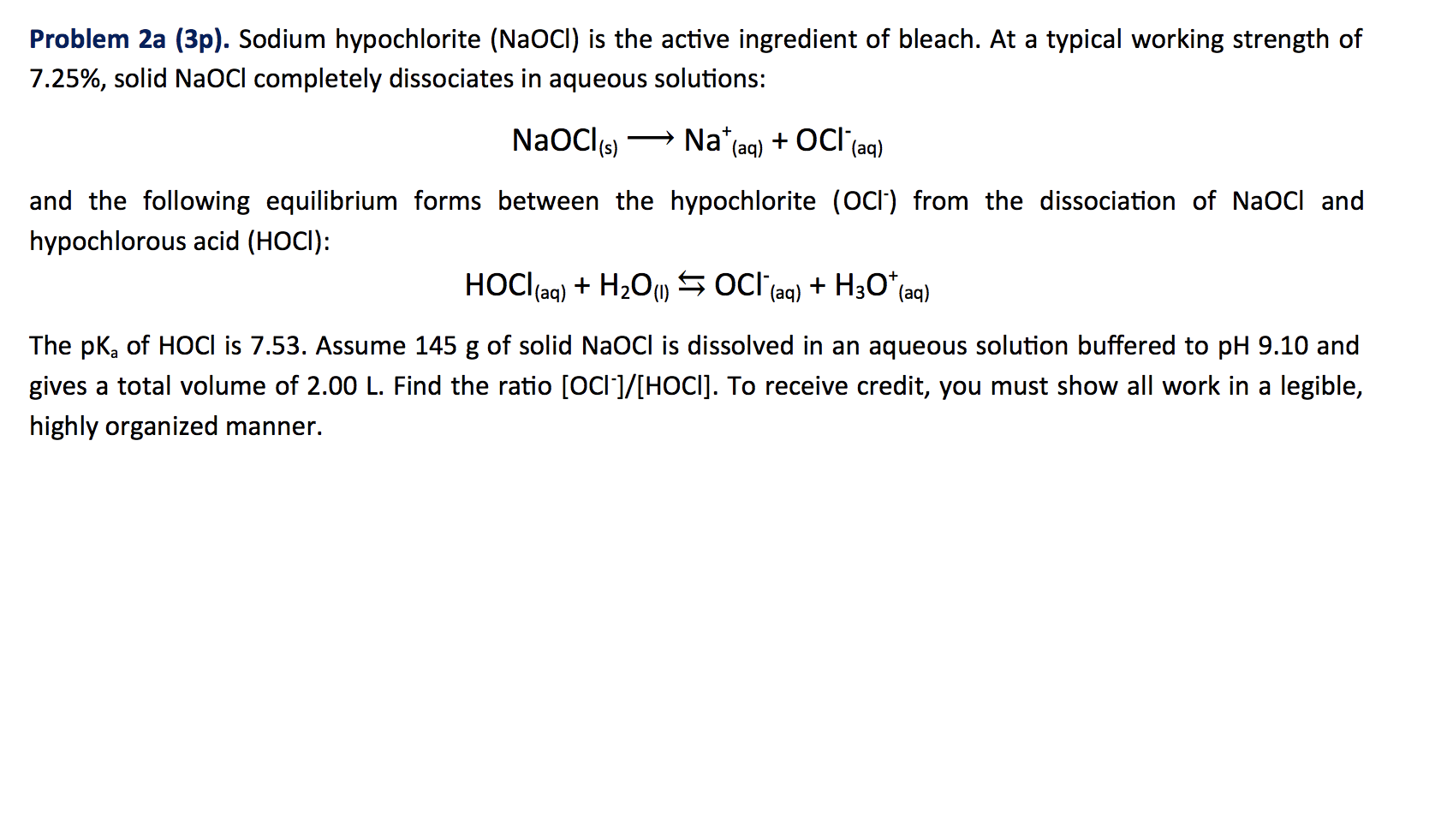
Sodium hypochlorite, NaClO is the active ingredient in many bleaches. Calculate the ratio of the concentration of ClO^- and HClO in a bleach having a pH adjusted to 6.50 by using strong

Experiment 19: OXIDATION OF 9-FLUORENOL. Objectives: To synthesize a ketone from a secondary alcohol using household bleach. To purify product using. - ppt download





![Is NaOCl Acidic or Basic [Acids and Bases] - YouTube Is NaOCl Acidic or Basic [Acids and Bases] - YouTube](https://i.ytimg.com/vi/HXJWALr3BEY/maxresdefault.jpg)