mass spectrum of N,N-dimethylmethanamine (trimethylamine) C3H9N (CH3)3N fragmentation pattern of m/z m/e

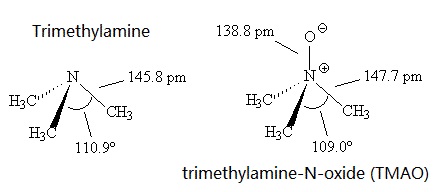
Welcome to Chem Zipper.com......: Why trimethylamine amine ( N(CH3)3) is tetrahedral while trisilyl amine (N(SiH3)3) planner.?

Trimethylamine, (CH3)3N, is a weak base that ionizes in aqueous solution: (CH3)3N(aq) + H2O(l) - Brainly.com

What is the Difference Between Dimethylamine and Trimethylamine | Compare the Difference Between Similar Terms
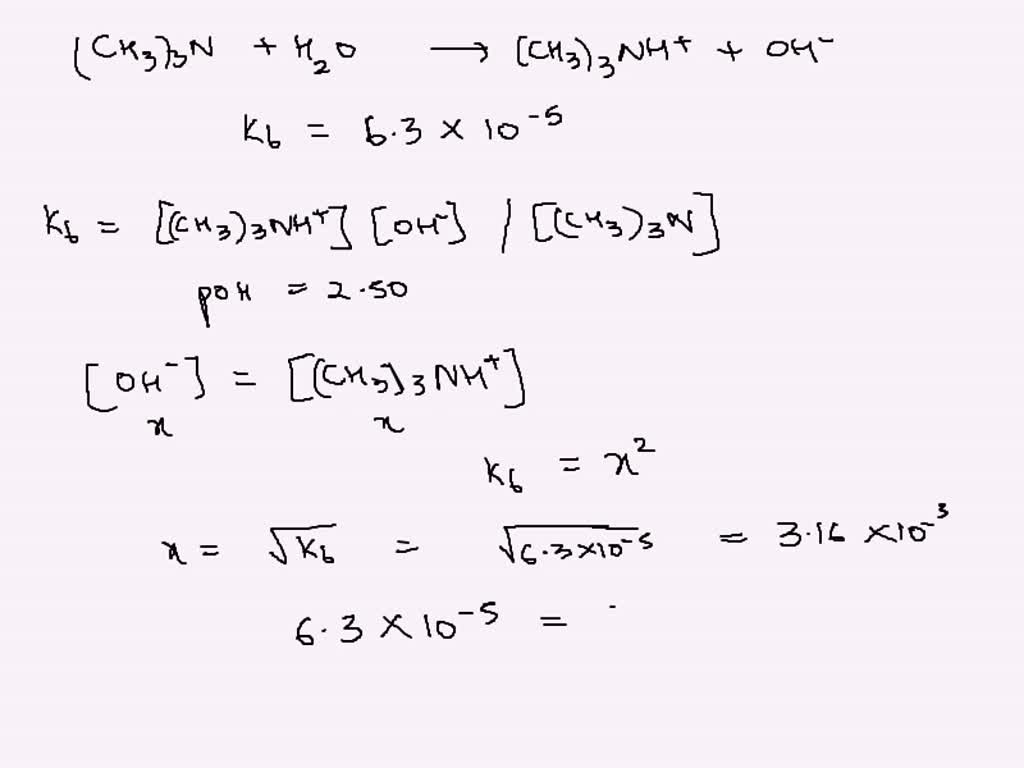
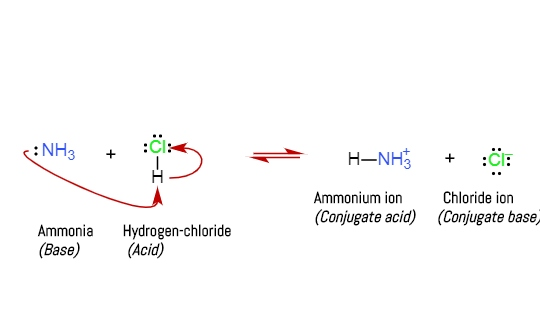
SOLVED: Trimethylamine, (CH3)3N, is a weak base (Kb = 6.3 × 10–5). What volume of this gas, measured at STP, must be dissolved in 2.5 L of solution to give that solution

Pls explain: Q Trisilyl amine is a weaker base than trimethyl amine because (a) in trisilyl amine pπ-pπ bonding - Chemistry - The p-Block Elements - 12586833 | Meritnation.com
✓ Solved: Base Ionization Trimethylamine, (CH3)3N, is a gas with a fishy, ammonialike odor. An aqueous...



![ANSWERED] Trimethylamine, (CH3)3N, is a weak base w... - Physical Chemistry ANSWERED] Trimethylamine, (CH3)3N, is a weak base w... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/73933115-1659733640.5428739.jpeg)